Iron Casting
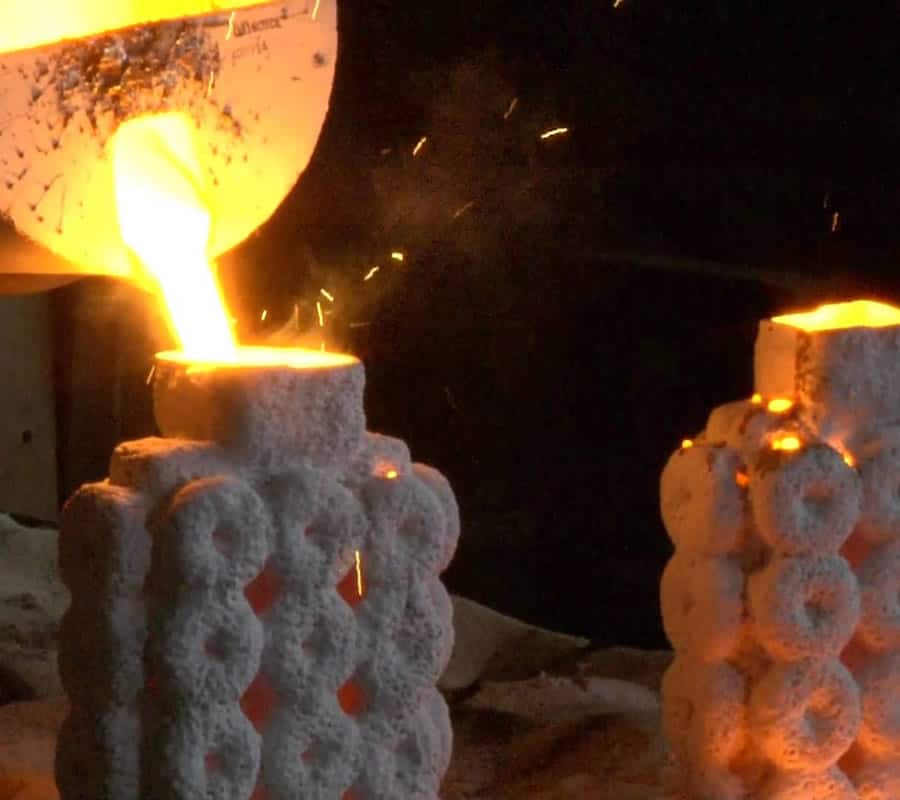
Read reviews and stay informed with product news articles. Whether you are looking for manufacturers of cast iron, ductile iron castings, and grey iron castings of. Welcome to Waupaca Foundry. Our company is the world's largest manufacturer of grey iron, ductile iron, compacted graphite, austempered and high-strength ductile iron castings. We take pride in producing best-in-class iron castings.
.Cast iron is a group of - with a carbon content greater than 2%. Its usefulness derives from its relatively low melting temperature.
The alloy constituents affect its colour when fractured: white cast iron has impurities which allow cracks to pass straight through, has graphite flakes which deflect a passing crack and initiate countless new cracks as the material breaks, and has spherical graphite 'nodules' which stop the crack from further progressing.Carbon (C) ranging from 1.8 to 4 wt%, and (Si) 1–3 wt%, are the main alloying elements of cast iron. Iron alloys with lower carbon content are known as.Cast iron tends to be, except for. With its relatively low melting point, good fluidity, excellent, resistance to deformation and, cast irons have become an with a wide range of applications and are used in, machines and parts, such as, and cases. It is resistant to damage by.The earliest cast-iron artefacts date to the 5th century BC, and were discovered by in what is now in China. Cast iron was used in ancient China for warfare, agriculture, and architecture.
During the 15th century, cast iron became utilized for cannon in, France, and in England during the. The amounts of cast iron used for cannon required large scale production. The first cast-iron bridge was built during the 1770s by, and is known as in,.
Cast iron was also used in the. Contents.Production Cast iron is made from, which is the product of melting iron ore in a. Cast iron can be made directly from the molten pig iron or by re-melting, often along with substantial quantities of iron, steel, limestone, carbon (coke) and taking various steps to remove undesirable contaminants.
And may be burnt out of the molten iron, but this also burns out the carbon, which must be replaced. Depending on the application, carbon and silicon content are adjusted to the desired levels, which may be anywhere from 2–3.5% and 1–3%, respectively. If desired, other elements are then added to the melt before the final form is produced by.Cast iron is sometimes melted in a special type of known as a, but in modern applications, it is more often melted in electric or electric arc furnaces. After melting is complete, the molten cast iron is poured into a holding furnace or ladle.Types Alloying elements. Iron-cementite meta-stable diagramCast iron's properties are changed by adding various alloying elements,. Next to, is the most important alloyant because it forces carbon out of solution.
A low percentage of silicon allows carbon to remain in solution forming iron carbide and the production of white cast iron. A high percentage of silicon forces carbon out of solution forming graphite and the production of grey cast iron. Other alloying agents, and counteracts silicon, promotes the retention of carbon, and the formation of those carbides. Nickel and copper increase strength, and machinability, but do not change the amount of graphite formed. The carbon in the form of results in a softer iron, reduces shrinkage, lowers strength, and decreases density., largely a contaminant when present, forms, which prevents the formation of graphite and increases. The problem with sulfur is that it makes molten cast iron viscous, which causes defects. To counter the effects of sulfur, is added because the two form into instead of iron sulfide.
The manganese sulfide is lighter than the melt, so it tends to float out of the melt and into the. The amount of manganese required to neutralize sulfur is 1.7 × sulfur content + 0.3%.
If more than this amount of manganese is added, then forms, which increases hardness and, except in grey iron, where up to 1% of manganese increases strength and density.is one of the most common alloying elements because it refines the and graphite structure, improves toughness, and evens out hardness differences between section thicknesses. Is added in small amounts to reduce free graphite, produce chill, and because it is a powerful stabilizer; nickel is often added in conjunction. A small amount of can be added as a substitute for 0.5% chromium. Is added in the ladle or in the furnace, on the order of 0.5–2.5%, to decrease chill, refine graphite, and increase fluidity.
Is added on the order of 0.3–1% to increase chill and refine the graphite and pearlite structure; it is often added in conjunction with nickel, copper, and chromium to form high strength irons. Is added as a degasser and deoxidizer, but it also increases fluidity.
0.15–0.5% is added to cast iron to stabilize cementite, increase hardness, and increase resistance to and heat. 0.1–0.3% helps to form graphite, deoxidize, and increase fluidity.In malleable iron melts, is added, on the scale of 0.002–0.01%, to increase how much silicon can be added.
She could not give me a refund, kept talking about how I had used the room (for half an hour). I had to inform her that as an employee of the hotel she was the representative of that establishment and sort of the only option I as a customer had! She was wondering why I was talking to her about this!  Had I got a refund, ticked off as I was, that would probably have been the end of it, but no such luck.
Had I got a refund, ticked off as I was, that would probably have been the end of it, but no such luck.
In white iron, is added to aid in the production of malleable iron; it also reduces the coarsening effect of bismuth. Grey cast iron. Main article:Grey cast iron is characterised by its graphitic microstructure, which causes fractures of the material to have a grey appearance. It is the most commonly used cast iron and the most widely used cast material based on weight. Most cast irons have a chemical composition of 2.5–4.0% carbon, 1–3% silicon, and the remainder iron. Grey cast iron has less and than steel, but its is comparable to low- and medium-carbon steel. These mechanical properties are controlled by the size and shape of the graphite flakes present in the microstructure and can be characterised according to the guidelines given by the.
White cast iron White cast iron displays white fractured surfaces due to the presence of an iron carbide precipitate called cementite. With a lower silicon content (graphitizing agent) and faster cooling rate, the carbon in white cast iron precipitates out of the melt as the phase, Fe 3C, rather than graphite.
The cementite which precipitates from the melt forms as relatively large particles. As the iron carbide precipitates out, it withdraws carbon from the original melt, moving the mixture toward one that is closer to eutectic, and the remaining phase is the lower iron-carbon (which on cooling might transform to ). These eutectic carbides are much too large to provide the benefit of what is called precipitation hardening (as in some steels, where much smaller cementite precipitates might inhibit by impeding the movement of through the pure iron ferrite matrix).
Rather, they increase the bulk hardness of the cast iron simply by virtue of their own very high hardness and their substantial volume fraction, such that the bulk hardness can be approximated by a rule of mixtures. In any case, they offer at the expense of.
Since carbide makes up a large fraction of the material, white cast iron could reasonably be classified as a. White iron is too brittle for use in many structural components, but with good hardness and abrasion resistance and relatively low cost, it finds use in such applications as the wear surfaces ( and ) of, shell liners and in and, balls and rings in, and the teeth of a 's digging bucket (although cast medium-carbon martensitic steel is more common for this application).It is difficult to cool thick castings fast enough to solidify the melt as white cast iron all the way through. However, rapid cooling can be used to solidify a shell of white cast iron, after which the remainder cools more slowly to form a core of grey cast iron.
The resulting casting, called a chilled casting, has the benefits of a hard surface with a somewhat tougher interior.High-chromium white iron alloys allow massive castings (for example, a 10-tonne impeller) to be sand cast, as the chromium reduces cooling rate required to produce carbides through the greater thicknesses of material. Chromium also produces carbides with impressive abrasion resistance. These high-chromium alloys attribute their superior hardness to the presence of chromium carbides. The main form of these carbides are the eutectic or primary M 7C 3 carbides, where 'M' represents iron or chromium and can vary depending on the alloy's composition. The eutectic carbides form as bundles of hollow hexagonal rods and grow perpendicular to the hexagonal basal plane.
The hardness of these carbides are within the range of 1500-1800HV. Malleable cast iron. Main article:Malleable iron starts as a white iron casting that is then for a day or two at about 950 °C (1,740 °F) and then cooled over a day or two. As a result, the carbon in iron carbide transforms into graphite and ferrite plus carbon (austenite). The slow process allows the to form the graphite into spheroidal particles rather than flakes.

Due to their lower, the spheroids are relatively short and far from one another, and have a lower vis-a-vis a propagating crack. They also have blunt boundaries, as opposed to flakes, which alleviates the stress concentration problems found in grey cast iron. In general, the properties of malleable cast iron are more like those of. There is a limit to how large a part can be cast in malleable iron, as it is made from white cast iron.Ductile cast iron. Main article:Developed in 1948, nodular or ductile cast iron has its graphite in the form of very tiny nodules with the graphite in the form of concentric layers forming the nodules.
As a result, the properties of ductile cast iron are that of a spongy steel without the stress concentration effects that flakes of graphite would produce. The carbon percentage present is 3-4% and percentage of silicon is 1.8-2.8%.Tiny amounts of 0.02 to 0.1%, and only 0.02 to 0.04% added to these alloys slow the growth of graphite precipitates by bonding to the edges of the graphite planes. Along with careful control of other elements and timing, this allows the carbon to separate as spheroidal particles as the material solidifies. Cast-iron plate on grand pianoCast iron and wrought iron can be produced unintentionally when smelting copper using iron ore as a flux.: 47–48The earliest cast-iron artifacts date to the 5th century BC, and were discovered by archaeologists in what is now modern, Jiangsu in China. This is based on an analysis of the artifact's microstructures.Because cast iron is comparatively brittle, it is not suitable for purposes where a sharp edge or flexibility is required. It is strong under compression, but not under tension. Cast iron was invented in China in the 5th century BC and poured into molds to make ploughshares and pots as well as weapons and pagodas.
Although steel was more desirable, cast iron was cheaper and thus was more commonly used for implements in ancient China, while or steel was used for weapons. The Chinese developed a method of cast iron by keeping hot castings in an oxidizing atmosphere for a week or longer in order to burn off some carbon near the surface in order to keep the surface layer from being too brittle.: 43In the west, where it did not become available until the 15th century, its earliest uses included cannon and shot. Initiated the casting of in England. Soon, English iron workers using developed the technique of producing cast-iron cannons, which, while heavier than the prevailing bronze cannons, were much cheaper and enabled England to arm her navy better. The technology of cast iron was transferred from China. Al-Qazvini in the 13th century and other travellers subsequently noted an iron industry in the Mountains to the south of the.
This is close to the, so that the use of technology derived from China is conceivable. The of the continued producing cast irons until the 1760s, and armament was one of the main uses of irons after the.Cast-iron pots were made at many English blast furnaces at the time. In 1707, patented a method of making pots (and kettles) thinner and hence cheaper than his rivals could.
This meant that his furnaces became dominant as suppliers of pots, an activity in which they were joined in the 1720s and 1730s by a small number of other -fired blast furnaces.Application of the steam engine to power blast bellows (indirectly by pumping water to a waterwheel) in Britain, beginning in 1743 and increasing in the 1750s, was a key factor in increasing the production of cast iron, which surged in the following decades. In addition to overcoming the limitation on water power, the steam-pumped-water powered blast gave higher furnace temperatures, which allowed the use of higher lime ratios, enabling the conversion from charcoal, supplies of wood for which were inadequate, to.: 122 Cast-iron bridges. See also:The use of cast iron for structural purposes began in the late 1770s, when built, although short beams had already been used, such as in the blast furnaces at Coalbrookdale. Other inventions followed, including one patented. Cast-iron bridges became commonplace as the gathered pace. Adopted the material for his bridge upstream at, and then for, a canal trough at on the. It was followed by the and the, both of which remain in use following the recent restorations.The best way of using cast iron for bridge construction was by using, so that all the material is in compression.
Cast iron, again like masonry, is very strong in compression. Wrought iron, like most other kinds of iron and indeed like most metals in general, is strong in tension, and also – resistant to fracturing.
The relationship between wrought iron and cast iron, for structural purposes, may be thought of as analogous to the relationship between wood and stone.Cast-iron beam bridges were used widely by the early railways, such as the Water Street Bridge in 1830 at the terminus of the, but problems with its use became all too apparent when a new bridge carrying the across the in collapsed killing five people in May 1847, less than a year after it was opened. The was caused by excessive loading at the centre of the beam by a passing train, and many similar bridges had to be demolished and rebuilt, often in.
The bridge had been badly designed, being trussed with wrought iron straps, which were wrongly thought to reinforce the structure. The centres of the beams were put into bending, with the lower edge in tension, where cast iron, like, is very weak.Nevertheless, cast iron continued to be used in inappropriate structural ways, until the disaster of 1879 cast serious doubt on the use of the material. Crucial lugs for holding tie bars and struts in the Tay Bridge had been cast integral with the columns, and they failed in the early stages of the accident. In addition, the bolt holes were also cast and not drilled. Thus, because of casting's draft angle, the tension from the tie bars was placed on the hole's edge rather than being spread over the length of the hole.
The replacement bridge was built in wrought iron and steel.Further bridge collapses occurred, however, culminating in the of 1891. Thousands of cast-iron rail were eventually replaced by steel equivalents by 1900 owing to the widespread concern about cast iron under bridges on the rail network in Britain. Main article:Cast-iron, pioneered in mill buildings, enabled architects to build multi-storey buildings without the enormously thick walls required for masonry buildings of any height. They also opened up floor spaces in factories, and sight lines in churches and auditoriums.
By the mid 19th century, cast iron columns were common in warehouse and industrial buildings, combined with wrought or cast iron beams, eventually leading to the development of steel-framed skyscrapers. Cast iron was also used sometimes for decorative facades, especially in the United States, and the district of New York has numerous examples. It was also used occasionally for complete prefabricated buildings, such as the historic in.Textile mills Another important use was in. The air in the mills contained flammable fibres from the cotton, or being spun. As a result, textile mills had an alarming propensity to burn down. The solution was to build them completely of non-combustible materials, and it was found convenient to provide the building with an iron frame, largely of cast iron, replacing flammable wood.
The first such building was at in, Shropshire. Many other warehouses were built using cast-iron columns and beams, although faulty designs, flawed beams or overloading sometimes caused building collapses and structural failures.During the Industrial Revolution, cast iron was also widely used for frame and other fixed parts of machinery, including spinning and later weaving machines in textile mills. Cast iron became widely used, and many towns had producing industrial and agricultural machinery.See also.